A Historic Leap Forward: FDA Approves First SCI-Specific Therapy
April 4, 2025
The Christopher & Dana Reeve Foundation is thrilled to celebrate a groundbreaking milestone in SCI treatment announced by our industry partner,... Read MoreOur blog offers stories from the community, updates from the Reeve Foundation, and breaking news on research.
The opinions expressed in these blogs are the author’s own and do not necessarily reflect the views of the Christopher & Dana Reeve Foundation.
.jpg)
April 4, 2025
The Christopher & Dana Reeve Foundation is thrilled to celebrate a groundbreaking milestone in SCI treatment announced by our industry partner,... Read More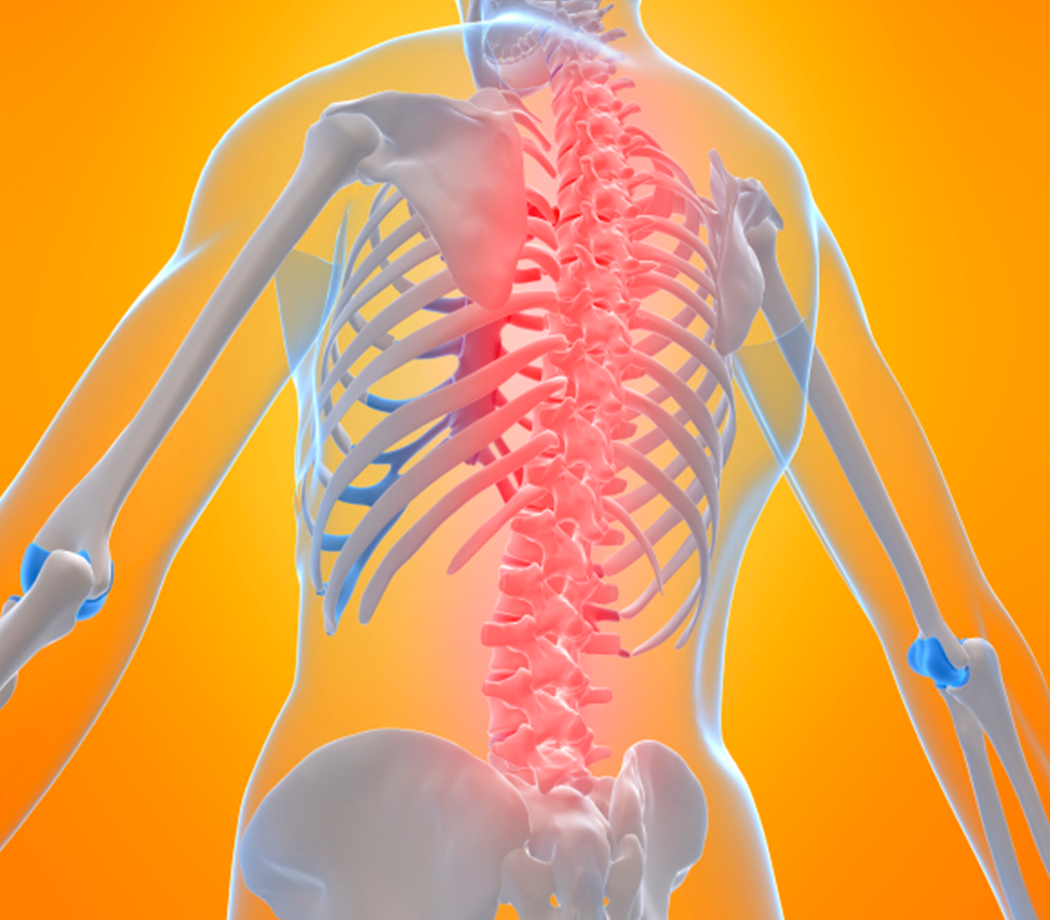
April 2, 2025
Unlike large pharmaceutical companies, small biotech companies and academic labs often lack the necessary resources to conduct essential early... Read More-1.png)
January 24, 2025
The Christopher & Dana Reeve Foundation hosted a panel discussion about the recent FDA market authorization for the ONWARD ARCEX System in the US.... Read More
February 28, 2024
Contributed by Lineage Cell Therapeutics Each year, spinal cord injury (SCI) affects an estimated 18,000 individuals in the United States, with less... Read More
November 13, 2023
Carta del Dr. Marco Baptista, Director Científico de la Fundación Reeve Read More
November 8, 2023
Los implantes espinales están logrando que lo imposible se vuelva posible para participantes con lesiones de la médula espinal Read More
September 27, 2023
Spinal Implants Are Making the Impossible Possible for Spinal Cord Injury Participants Read More
September 27, 2023
A Letter from the Reeve Foundation's, Chief Scientific Officer, Dr. Marco Baptista Read More
September 27, 2023
Scientists Learn to Share Data — A Giant Step Forward in Advancing SCI Research Read MoreThe opinions expressed in these blogs are the author's own and do not necessarily reflect the views of the Christopher & Dana Reeve Foundation.