Epidural Stimulation for Hand Function: It’s What’s New - Reeve Foundation
When I’m reading a peer-reviewed research paper, I often find myself translating as I go. Here, for example, is how I deciphered some language from a Yury Gerasimenko paper published back in the late fall of 2016.
Dr. Gerasimenko: Paralysis of the upper limbs from spinal cord injury results in an enormous loss of independence in an individual’s daily life. Meaningful improvement in hand function is rare after one year of tetraparesis.
Me: Damage to the upper part of your spinal cord often means your hands don’t work properly, which means you can’t do necessary things for yourself. This might change during the first year or so, but after that whatever you have left is all you’re ever going to have.
This isn’t news to anyone reading this blog or to anyone reading that paper, but it has to be stated anyway because it sets the stage for what comes next.
Dr. Gerasimenko: Herein we show that epidural stimulation (ES) can be applied to the chronic injured human cervical spinal cord to promote volitional hand function.
Me: Epidural stimulation (described here as it’s been used to promote recovery of movement in the legs) also works in the hands … even in injuries that are long past the one-year mark.
That was definitely news. Until this paper was published, all we knew for sure was that implanting a stimulator into the lumbar area had the effect of enabling people with chronic injuries to move muscles that had not moved since before the injury. Dr. Gerasimenko and his colleagues were saying that they’d shown that if the implant was placed higher, it had a similar effect on the hands and fingers.
Specifically, they described what happened when researchers used epidural stimulation on two people with cervical injuries, one at C5 and the other at C6. Both were classified as AIS B at the time of the study, meaning they had some sensation below their injury levels, but no detectable movement at all. Both were at least 18 months post injury. They also both suffered from chronic pain in their shoulders and upper arms – pain that hadn’t been treatable by anything they had tried.
It’s interesting. The reason epidural stimulators were originally approved by the FDA is that they’re effective in treating certain kinds of pain. They weren’t developed to enable movement after spinal cord injury at all; they were supposed to be an alternative to drugs for chronic pain. The studies that showed they were effective, unfortunately, did not show that stimulators helped people with neuropathic pain after spinal cord injury. And yet:
Dr. Gerasimenko: For both subjects, pain improvement occurs almost immediately within 15 minutes of initial stimulation prior to these experiments … the stimulation parameters for pain control are different with respect to electrode location, frequency, and amplitude. ES as used in this study for improvement of motor performance had no impact on pain …
Me: The original studies that showed epidural stimulators don’t help with neuropathic pain didn’t test for the kind of pain these patients had in their arms and shoulders, which seems like a tragedy given that the patients in Dr. Gerasimenko’s study felt better right away. (I bet that was a welcome surprise.) Also, it turned out just as the scientists hoped -- that when you adjust the stimulator dials with an eye not to numbing pain but rather to improving hand function, you can get results without causing new, different, or more pain.
How exactly did this work? The “stimulator” in this case is a set of 16 electrodes that deliver a current – a carefully managed flow of electrons. That current isn’t a steady stream like a long blast of water from a hose; it’s more like the way water comes out of a lawn sprinkler, in predictable little bursts. The stimulator can be adjusted along three separate specifications: amplitude, (how strong) frequency (how fast) and pulse width (how long each individual burst lasts). The electrodes in this study were implanted just outside the membrane that protects the cord (called the dura). They formed an array between C4 and T1, which means from about the center of the neck down to a spot at the top of the shoulders.
With the electrodes in place, the researchers then adjusted the current so that it gave the patients the maximum possible ability to respond with the minimum of current. It’s important to remember that this is not direct stimulation of muscles. It’s micro-stimulation of the cord itself, and it works.
Sort of.
These subjects began with four straight months of testing and training without the stimulators, which is necessary to make sure the training itself isn’t the cause of any improvements that might happen once they have their stimulators; there has to be a baseline . The pre-implantation training involved coming to the lab every week and trying to squeeze and release the bar on the same handgrip device that would later be part of the post-implantation study.
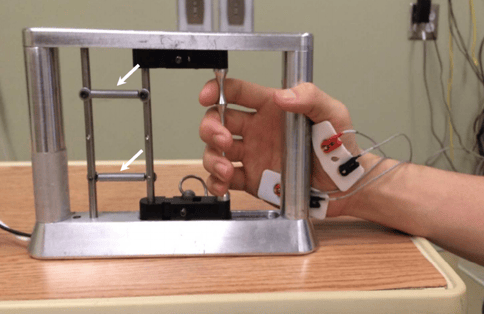
Dr. Gerasimenko: In both subjects, there were no consistent improvements during this ‘lead in’ phase that could be attributed to training … these results are consistent with severely cervical injured individuals.
Me: Training alone did nothing to help these patients.
What about after they had the stimulators?
Dr. Gerasimenko: Average maximum grip force increased cumulatively over multiple sessions with ES following implantation in both subjects … ES also resulted in some long-lasting effects in improved self-care and mobility due to improved hand and arm function. The subjects’ self-care was impacted in all subcategories (feeding, bathing, dressing, and grooming) and mobility in bed and transfers.
Me: The stimulators caused what seems to be a small but permanent improvement in these subjects’ ability to use their hands, and that improvement – however small – meant more independence.
That, friends, is very good news. Next time we’ll be talking about a much less invasive (but just as effective) kind of stimulator.
Stay tuned.
Join Our Movement
What started as an idea has become a national movement. With your support, we can influence policy and inspire lasting change.
Become an Advocate